This guidance will evolve rapidly; please see the BHIVA website for the latest version
Monday 11 January 2021
Introduction
The UK was the first country to approve the use of a SARS-CoV-2 vaccine, Pfizer’s BioNTech COVID-19 vaccine, to prevent COVID-19 disease on 2nd December 2020. The AstraZeneca/Oxford vaccine was also approved for use in December 2020, with vaccinations beginning on 4th January 2021, and the Moderna vaccine on 8th January 2021 with first UK doses expected Spring 2021.
The Medicines and Healthcare Regulatory Authority (MHRA) is a branch of the Department of Health and Social Care, regulates medicines in the UK and monitors safety once a product has been approved. The MHRA, with advice from the independent advisory body, the Commission on Human Medicines, make recommendations for approval of medicines in the UK.
The Joint Committee on Vaccination and Immunisation (JCVI) is an independent group of experts who advise the Government health departments in the four UK nations on immunisations and the prevention of infectious disease. The JCVI advise how best to get vaccines to the public and where further research or surveillance may be required; their priority for COVID-19 vaccinations is to reduce mortality and serious disease, and protect the NHS and social care system.
Are the vaccines live?
No, none of the SARS-CoV-2 vaccines in development are live. Of the two most advanced vaccines:
Pfizer’s BioNTech and Moderna’s mRNA-1273 vaccine are both synthetic mRNA vaccines
AstraZeneca’s ChAdOx1 vaccine is replication-defective chimpanzee adenovirus-vectored vaccine expressing a sequence of the SARS-CoV-2 gene
Who will be prioritised to get the vaccine early?
The JCVI has recommended nine priority groups based on age, residency in care institutions, occupation and the presence of medical conditions or treatments that suppress the immune system. These priority groups are estimated to include 99% of the people at risk of dying from COVID-19 and are outlined in the Green Book chapter on COVID-19: https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a
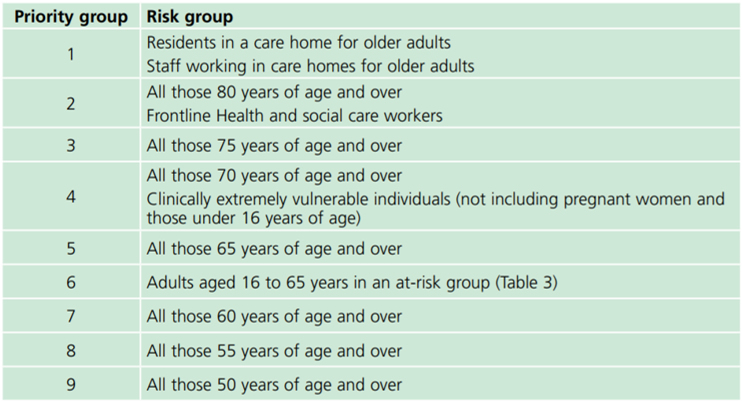
Priority group 4: Clinically Extremely Vulnerable
People considered clinically extremely vulnerable include those with an organ transplant, or some cancers or those who are on medication that affects the immune system.
For people with HIV we advise that anybody with a CD4 count less than 50 cells/mm3, or who has had an opportunistic illness in the last 6 months, should be considered clinically extremely vulnerable.
All people, particularly those with a CD4 count in the range 50-200 cells/mm3, with additional risk factors should be assessed on an individual basis and, if deemed extremely clinically vulnerable, can be added to this category. Additional risk factors to consider include:
- Detectable viral load
- Low nadir CD4 count
- Additional co-morbidities associated with moderate risk:
- Non-severe lung condition (e.g. asthma, COPD, emphysema or bronchitis)
- Heart disease (e.g. heart failure)
- Diabetes
- Chronic kidney disease
- Liver disease (such as hepatitis)
- A neurological condition (e.g. Parkinson's disease, motor neurone disease, multiple sclerosis or cerebral palsy)
- Another immunosuppressive condition or medication (e.g. low dose steroids)
- Severe obesity (a BMI of 40 or above).People considered to be extremely clinically vulnerable will not necessarily have been coded as such, unless they have another condition in this category. They may therefore need to be added to this list. Each Trust will have someone allocated to submit data to the Strategic Data Collection Service (SDCS) https://datacollection.sdcs.digital.nhs.uk/. For further information about the Shielded Patient List (SPL): https://digital.nhs.uk/coronavirus/shielded-patient-list
Additional information can be found here:
England: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/whos-at-higher-risk-from-coronavirus/ & https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19#cev
Scotland: https://www.gov.scot/publications/covid-shielding/pages/highest-risk-classification/
Wales: https://gov.wales/guidance-protecting-people-defined-medical-grounds-extremely-vulnerable-coronavirus-covid-19
Northern Ireland: https://www.nidirect.gov.uk/articles/coronavirus-covid-19-guidance-clinically-extremely-vulnerable-and-vulnerable-people
Ireland: https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/guidance/vulnerablegroupsguidance/
Priority group 6: people with underlying health conditions which put them at higher risk of serious disease and mortality
These conditions are outlined in the Green Book and include all people with HIV.
Is the vaccine safe for people with HIV?
The patient information leaflets for the Pfizer/BioNTech, AstraZeneca/Oxford COVID-19 and Moderna vaccines all list immunodeficiency under the warnings and precautions for use, with Pfizer using HIV as an example, advising affected patients to tell their doctor/nurse/pharmacist before vaccination.
It is important to state that this is not based on concerns over safety. There is currently no evidence to suggest a higher risk of side effects in people with HIV. The guidance is mainly based on the fact that there are limited data for people with immune deficiency and/or HIV.
It is possible that people with HIV will produce a weaker response to the COVID-19 vaccines, but the vaccines are still expected to be protective, and are recommended.
The Department of Health guidance is clear that people with HIV, regardless of CD4 count, should have a COVID-19 vaccine. None of the currently available COVID-19 vaccines is live so the vaccines cannot cause COVID-19. The Pfizer/BioNTech vaccine is based on mRNA whereas the AstraZeneca/Oxford vaccine contains non-replicable adenovirus. Both vaccines are considered safe for people with suppressed immune systems; similar adenovirus vaccines have been studied in populations where HIV is common and have not been associated with harm.
BHIVA has had contact with Pfizer to raise concern about specifically warning about HIV in the patient information and will update you with the company’s response.
Further information for HIV patients is available at iBase: https://i-base.info/qa/16330 which is updated regularly.
Further information
Further information for health care professionals about the Pfizer vaccine, pending formal MHRA approval, can be accessed here: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940565/
Information_for_Healthcare_Professionals_on_Pfizer_BioNTech_COVID-19_vaccine.pdf
Notes: Safety data will be produced rapidly as the vaccine is rolled out. Please see MHRA website for the latest information.
For further information, please contact [email protected] or for media enquiries, please contact Jo Josh at [email protected] or +44 (0)7306 391875.



